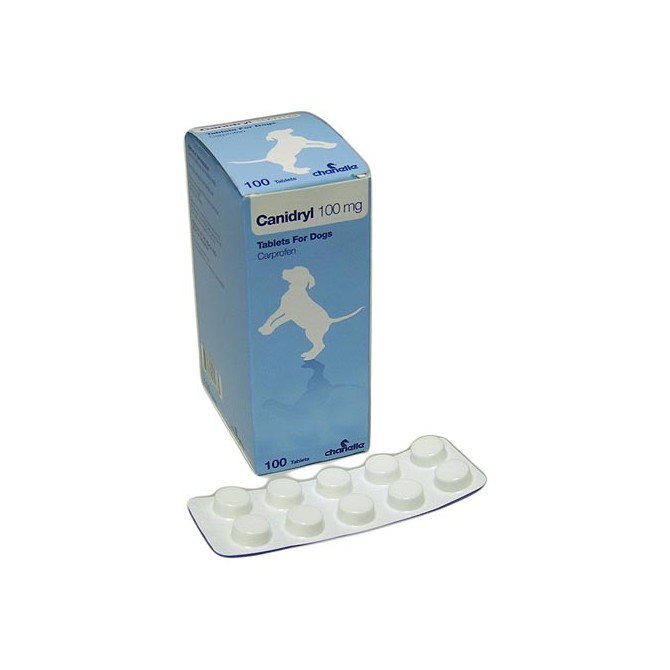
Canidryl Flavour Snap Tablet 100mg - per Tablet
372900
Canidryl Flavour Tablet 100mg - per Tablet. VET PRESCRIPTION REQUIRED
Product Features
- Pack Size: - Sold Individually
- Target Animal: - Dog (Canine)
- Related Condition: - For use in the reduction of inflammation and pain caused by musculo-skeletal disorders and degenerative joint disease in dogs
- Pet Prescription Required?: - Yes (For UK Orders)
- Active Ingredient: - 100mg Carprofen
- Product Name: - Canidryl Flavour Tablet 100mg - per Tablet
More Information
Description
Canidryl Flavour Tablets - 100mg
Canidryl may only be supplied with a valid veterinary prescription issued by your vet. You should only purchase Canidryl if you have or are in the process of arranging such a prescription. See information bar for further details.
Canidryl Flavour Tablets are grilled-meat flavoured tablets containing Carprofen 100mg/tablet
A plain round flat bevelled edge white tablet with a breakline on one side. Canidryl Flavour Tablets can be broken into equal halves.
For use in the reduction of inflammation and pain caused by musculo-skeletal disorders and degenerative joint disease in dogs. As a follow up to parenteral analgesia in the management of post-operative pain for soft tissue surgery.
Contra-indications
Do not use Canidryl in cats. Do not use Canidryl in case of hypersensitivity to active substance or to any of the excipients. Do not use Canidryl in dogs suffering from cardiac, hepatic or renal disease, where there is a possibility of gastro-intestinal ulceration or bleeding, or where there is evidence of a blood dyscrasia. Special Precautions for use in Animals Use in dogs less than 6 weeks of age, or in aged dogs, may involve additional risk. If such a use cannot be avoided, dogs may require careful clinical management. Avoid use of Canidryl in any dehydrated, hypovolaemic or hypotensive dog, as there is a potential risk of increased renal toxicity. Concurrent administration of potential nephrotoxic drugs should be avoided. NSAIDs can cause inhibition of phagocytosis and hence in the treatment of inflammatory conditions associated with bacterial infection, appropriate concurrent antimicrobial therapy should be instigated. Do not administer other NSAIDs concurrently or within 24 hours of each other. Some NSAIDs may be highly bound to plasma proteins and compete with other highly bound drugs, which can lead to toxic effects.
Special Precautions to be taken by the Person Administering the Product to Animals In the event of accidental ingestion of the Canidryl, seek medical advice and show the doctor the package leaflet. Wash hands after handling Canidryl. Adverse reactions (Frequency and Seriousness) Typical undesirable effects associated with NSAIDs, such as vomiting, soft faeces/diarrhoea, faecal occult blood, loss of appetite and lethargy have been reported. These adverse reactions occur generally within the first treatment week and are in most cases transient and disappear following termination of the treatment but in very rare cases may be serious or fatal. If adverse reactions occur, use of the product should be stopped and the advice of a veterinarian should be sought.
As with other NSAIDs there is a risk of rare renal or idiosyncratic hepatic adverse events. Use During Pregnancy, lactation or lay Studies in laboratory species (rat and rabbit) have shown evidence of foetotoxic effects of carprofen at doses close to the therapeutic dose. The safety of the veterinary medicinal product has not been established during pregnancy and lactation. Do not use in pregnant or lactating bitches. Interaction with other medicinal products and other forms of interaction Carprofen must not be administered with glucocorticoids.
Refer also to "Special Precautions for Use". Amounts to be administered and administration route For oral administration. 4 mg carprofen per kg bodyweight per day. An initial dose of 4 mg carprofen per kg bodyweight per day given as a single daily dose or in two equally divided doses may, subject to clinical response, be reduced after 7 days to 2 mg carprofen/kg bodyweight/day given as a single dose. Duration of treatment will be dependant upon the response seen. Long term treatment should be under regular veterinary supervision. To extend analgesic and anti-inflammatory cover post-operatively, parenteral preoperative treatment may be followed with Carprofen tablets at 4mg/kg/day for 2 days. Do not exceed the stated dose.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements.
Legal category
POM-V (Pet Prescription Required)





