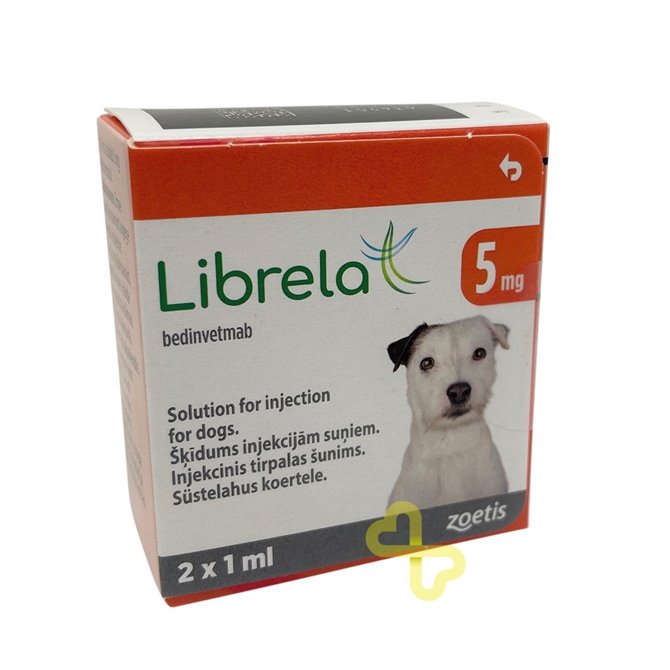
5mg Librela Solution for Dogs - 2 Vials
370927
5mg Librela is an innovative monthly drug for the alleviation of pain associated with osteoarthritis in dogs.
Librela 5mg is a breakthrough solution for dogs suffering from osteoarthritis pain. The treatment effectively relieves discomfort for a whole month, with a safety profile proven through rigorous testing. Librela works uniquely, targeting Nerve Growth Factor (NGF), a significant contributor to osteoarthritis pain, offering a different approach than Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
Librela 5mg uses monoclonal antibodies—immune system proteins that neutralise disease-causing molecules—mirroring naturally occurring antibodies. With minimal involvement of liver or kidneys, it’s a gentle option with a minimal gastrointestinal impact, reducing the potential for adverse reactions.
Librela is a refrigerated medication - We will send it via Royal Mail Special Delivery using insulated packaging and ice packs. A signature is required on delivery and it must be put straight into a fridge. We cannot guarantee same day dispatch for orders placed after 12pm. VETERINARY PRESCRIPTION REQUIRED.
Clinical trials demonstrated that dogs experienced decreased pain and increased mobility after just one injection. Delivered as a monthly injection at the clinic, Librela ensures the efficient management of osteoarthritis pain.
Librela 5mg contains a canine monoclonal antibody (mAb) which targets nerve growth factor (NGF). This inhibits NGF-mediated cell signalling which has been demonstrated to provide relief from pain associated with osteoarthritis.
Product Features
- Pack Size: - Pack of 2
- Target Animal: - Dog (Canine)
- Related Condition: - Osteoarthritis in Dogs
- Pet Prescription Required?: - Yes
- Active Ingredient: - Bedinvetmab
- Product Name: - Librela solution for injection for dogs
More Information
Description
5mg Librela - Helping to better manage a dog's arthritis
Librela offers a proven safety profile, with no known interactions with other medications. It was well-tolerated even when co-administered with other commonly used treatments, including parasiticides, antibiotics, vaccines, and RIMADYL®.
Librela is a versatile option suitable for long-term osteoarthritis pain management and those not tolerating NSAIDs. Ideal for dogs who may struggle with oral medication, it brings rapid relief that can be started and stopped as necessary.
Osteoarthritis, affecting 4 out of every 10 dogs, can greatly impact a dog's quality of life and mobility. With Librela, we can improve the lives of dogs suffering from osteoarthritis by managing their pain effectively and improving their quality of life.
Each vial of 1ml Librela contains 5mg bedinvetmab. Librela is a caninised monoclonal antibody.
Librela is used for the alleviation of pain associated with osteoarthritis in Dogs.
Dosage and administration
Librela is for subcutaneous use. The recommended dose of Librela is 0.5-1.0 mg/kg bodyweight, once a month.
For dogs weighing <5.0 kg aseptically withdraw 0.1 ml/kg from a single 5 mg/ml vial and administer subcutaneously. For dogs between 5 and 60 kg administer the entire content of the vial (1ml) according to the dose prescribed by your Vet. For dogs above 60 kg, the contents of more than one vial are required to administer a single dose. In those cases, withdraw the content from each required vial into the same syringe and administer as a single subcutaneous injection (2ml).
Contra-indications, warnings, etc.
Do not use Librela in cases of hypersensitivity to the active substance or to any of the excipients. Do not use Librela in dogs under 12 months. Do not use Librela in animals intended for breeding. Do not use Librela in pregnant or lactating animals.
Librela may induce transient or persistent anti-drug antibodies. The induction of such antibodies is uncommon and may have no effect or may result in a decrease in efficacy in animals that responded to treatment previously. If no or limited response is observed within one month after initial dosing, an improvement in response may be observed after administration of a second dose one month later. However, if the animal does not show a better response after the second dose, the veterinary surgeon should consider alternative treatments. The safety of the veterinary medicinal product has not been established during pregnancy and lactation or in breeding dogs. Laboratory studies with anti-NGF antibodies in cynomolgus monkeys have shown evidence of teratogenic and foetotoxic effects. In a laboratory study over a 2-week period in young, healthy dogs without osteoarthritis, this veterinary medicinal product had no adverse effect when concomitantly administered with a non-steroidal anti-inflammatory product (carprofen). There are no safety data on the concurrent long-term use of NSAIDs and Librela in dogs. In clinical trials in humans, rapidly progressive osteoarthritis has been reported in patients receiving humanised anti-NGF monoclonal antibody therapy. The incidence of these events increased with high doses and in those human patients that received long-term (more than 90 days) non-steroidal anti-inflammatory drugs (NSAIDs) concomitantly with an anti-NGF monoclonal antibody. Dogs have no reported equivalent of human rapidly progressive osteoarthritis. No other laboratory studies on the safety of concomitant administration of this veterinary medicinal product with other veterinary medicinal products have been conducted. No interactions were observed in field studies where this veterinary medicinal product was administered concomitantly with veterinary medicinal products containing parasiticides, antimicrobials, topical antiseptics with or without corticosteroids, antihistamines and vaccines. If a vaccine(s) is to be administered at the same time as treatment with this veterinary medicinal product, the vaccine(s) should be administered at a different site to that of Librela’s administration, to reduce any potential impact on immunogenicity of the vaccine. Do not mix with any other veterinary medicinal product. Mild reactions at the injection site (e.g. swelling and heat) may uncommonly be observed. The frequency of adverse reactions is defined using the following convention: - very common (more than 1 in 10 animals treated displaying adverse reaction(s)) - common (more than 1 but less than 10 animals in 100 animals treated) - uncommon (more than 1 but less than 10 animals in 1,000 animals treated) - rare (more than 1 but less than 10 animals in 10,000 animals treated) - very rare (less than 1 animal in 10,000 animals treated, including isolated reports). No adverse reactions, except mild reactions at the injection site, were observed in a laboratory overdose study when Librela was administered for 7 consecutive monthly doses at10 times the maximum recommended dose. In case of adverse clinical signs after an overdose the dog should be treated symptomatically. User warnings Hypersensitivity reactions, including anaphylaxis, could potentially occur in the case of accidental self-injection. Repeated self-administration may increase the risk of hypersensitivity reactions. The importance of Nerve Growth Factor in ensuring normal foetal nervous system development is well-established and laboratory studies conducted on non-human primates with human anti-NGF antibodies have shown evidence of reproductive and developmental toxicity. Pregnant women, women trying to conceive and breastfeeding women should take extreme care to avoid accidental self-injection. In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician. Pharmaceutical precautions
Store Librela in a refrigerator (2°C - 8°C). Do not freeze. Store in the original package. Protect from light. Shelf life after first opening the immediate packaging: use immediately Keep out of the sight and reach of children. For animal treatment only.
Legal category:
Librela is a POM-V (Pet Prescription Required)
You may also like

Arthri-Aid HA Powder ArthriAid - 400g
ArthriAid HA Powder for Dogs & Cats is a unique combination of high strength Glucosamine, Chondroitin and MSM plus essential co-factors that help encourage the manufacture of new cartilage and synovial fluid leading to easier joint mobility.
Help maintain your pet's joint function and mobility with ArthriAid HA Powder for Dogs & Cats. This unique formula is based on a blend of the natural nutrients Chondroitin, MSM, Glucosamine, DHA and essential co-factors to help encourage the production of new cartilage for dogs.
Arthri-Aid Omega Joint Supplement ArthriAid - 250ml
ArthriAid Omega for Dogs & Cats is an innovative combination of Glucosamine, Chondroitin and MSM plus essential cofactors that helps reduce stiffness, joint pain and discomfort in dogs. This unique formulation also helps to encourage the manufacture of new cartilage and synovial fluid leading to easier joint mobility.
250ml Arthri Aid

YuMOVE Chews Hip and Joint Supplement for Large Dogs - 30 Chews
YuMOVE One-a-Day Chewies' natural formula makes a tail-wagging difference in three ways: soothing stiffness, supporting long-term joint health and promoting mobility. They're proven to work within six weeks*, thanks to a powerful blend of ingredients that includes the world's top-strength, cold-extracted ActivEase Green Lipped Mussel.
BD Discardit 2ml Syringe with 21g Needle - each
2ml Syringe complete with 21 gauge needle. Suitable for injection of Librela, Cytopoint, Solensia etc.




